An Introduction to Fungi and Wild Mushroom Identification for Foragers
Responsibly foraging for edible mushrooms is a wonderful entry point into a wider appreciation of fungi – what it is, how it works, and all the wonderful roles it plays in the natural world. Nothing excites and intimidates novice foragers quite like fungi. The allure of gourmet species like ceps and chanterelles is usually partnered by a pervasive fear of fatally poisonous species like the death cap, funeral bell and destroying angel. Even experienced fungi foragers can struggle to progress their knowledge beyond a few easily recognised species. Often this means they are missing out on some great food, medicine and tools.
The themes discussed in this post are explored in greater depth in these three fungi webinars.
Here I will attempt to unpick this fascinating, often scary, but ultimately very rewarding world, by presenting a structure around which novice and intermediate fungi fans may advance their understanding of fungi lifecycle, learn to recognise important mushroom families, develop mindful foraging techniques, and feel safe in the process.
Even a general look at fungi could easily be the topic of a very thick book, not a few thousand words, so we need to be realistic…
1: Manage Your Expectations
A few years ago I was lucky enough to spend a day in the company of eminent Scottish mycologist, Liz Holden. We surveyed a 500m by 100m area of mixed beech and spruce forest in mid September. We found roughly 100 species of fungi – from large boletes and bracket fungi to minute species, barely visible to the untrained eye. With my 25 years of fairly unscientific interest in fungi, I could identify, with reasonable confidence, about half of these in the field using a guidebook. Liz (who’s knowledge is light years ahead of mine) was pretty confident, after consulting several guidebooks, on perhaps 70%.
She informed me that the remainder could not be definitively identified without a microscope.
Please take a moment to digest that, and refine your own expectations of what you might be able to confidently identify with your own level of experience. Note that you will most certainly need multiple guide books to progress your knowledge, and even with them, many species will defy identification without examination under a microscope. There are lots of excellent field guides available quite cheaply – please see my recommendations here.
I hope this wont put anyone off: there are some “easy wins” out there, but for the most part getting anywhere with fungi requires, like all worthwhile pastimes, investing some focussed, practical time in the field and at home. The journey can be frustrating, but the rewards are massive and the knowledge you gain could be life-changing, not to mention life-saving.

Liz Holden at work. Note that her field guide is ever-present. Liz used a wide variety of plastic bags and tubs to take samples for identification – not a whicker basket in sight!
2: Why Fungi Identification Is Often Difficult
The sheer number of different species of fungi can be bewildering. Its hard to place a precise number on just how many there are, as many are micro fungi – minute organisms that can’t be seen by the naked eye. New species are being discovered regularly. Better known micro fungi include the yeasts that ferment your bread and beer as well as penicillin and athletes foot. For the purposes of this article we will be focusing on macro fungi, the reproductive parts of which can be seen with the naked eye. There are approximately 7000 of these in the UK – more than there are flowering plants.
Struggling with mushroom identification?
Check out my online mentoring for 1-to-1 help developing your ID skills.
I use the term “reproductive parts” because the things we generally refer to as mushrooms or toadstools are the reproductive organs of cryptic, hidden organisms that can rarely be seen with the naked eye. This goes a long way to explaining the difficulties of fungi identification: imagine trying to identify animals, or even plants, by only looking at their reproductive parts!

Some fungi can look more like reproductive parts than others… These are stinkhorns (Phallus impudicus)
To further frustrate our attempts to get familiar with fungi, these “fruiting bodies” (they aren’t fruits as they don’t have ovaries, but the comparison is useful), appear quickly and can often start to decompose before they are fully formed. It can be difficult for beginners to know if they are looking at an immature, fully formed, or semi-decomposed specimen.

4 out of 5 of these ceps (Boletus edulis) are rotten and decomposing. Identification of these would be challenging for a beginner.
Add to this the short period of the year when most fungi are actually visible above ground, and its not surprising that most people struggle to understand them. Imagine if plants only appeared above ground as their seed casings for 1 month of the year during which they rapidly change shape. Would you be able to identify even the simplest with any confidence?

These are all the same species of fungi (Boletus edulis), picked at the same time, from the same area, but all displaying quite different forms. This variation within species can be challenging
Small wonder then that fungi have historically been widely overlooked, often misunderstood, and all-too-often abused. The UK in particular suffers from a deep-rooted mycophobia (fear of fungi) that the relatively recent upsurge of interest in edible species and the wider contribution of fungi to ecosystems and the health of our planet is just beginning to reverse.
Rules of Thumb for Identifying Fungi
It is understandable why many foraging novices pine for simple “rules of thumb” to tell poisonous from edible fungi. I’ve got to tell you straight from the outset: there aren’t any.
There are no dangerous colours, no tell-tale characteristics (like having a peelable cap), no easy and obvious warning signs. Imagining such simple solutions is more likely to see you poisoned. The sooner you can move beyond this false hope, and realise that identifying species accurately is the only way to stay safe, the sooner you will improve your skills and knowledge. Always remember that you are in no way obliged to eat a wild mushroom – if in doubt, leave it out and never munch on a hunch!
Read my blog: Mushrooms and Toadstools: What’s the Difference?
I have noticed the occasional book, and even some fearful and inexperienced instructors, that suggest the way to stay safe is to “never eat a gilled fungi”. While it is true that this will eliminate the possibility of eating a large proportion of seriously poisonous fungi, it certainly doesn’t cover all potentially harmful fungi and may lead to overconfidence. It’s also a bit like saying “don’t eat any plant with white flowers” and missing out on a wealth of delights. Worse than this, it tends to discourage interest in the wider world of fungi, and your identification skills will never improve. Resist tossing challenging ID’s over your shoulder, and invest a little time in observation and research.
3: Basic Fungi Science – Mycology
To overcome identification difficulties and lay strong foundations for a wider appreciation of fungi, it is important to form a clear understanding of what it actually is.
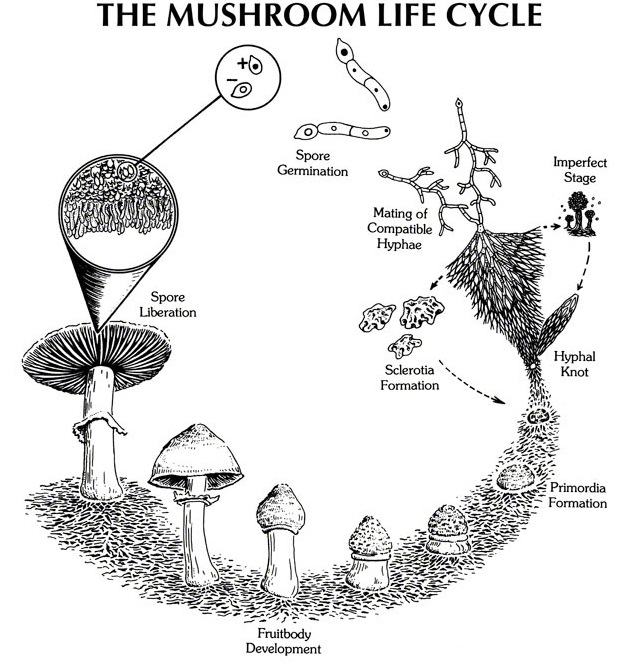
If we think of the visible structures of fungi as “fruiting bodies”, the parent structure from which these grow is hidden from view and consists of a mass of thin threads called hyphae, which permeate the particular species’ growing medium. Individually these are about one tenth the diameter of a human hair, unnoticeable to the human eye. They form complex networks that we refer to as mycelium. Usually even these networks are hard to see, but as the individual strands join and coalesce they can start to become visible. If you have kicked a pile of rotting leaves in the autumn and noticed fine, cottony threads running through them, this is mycelium. The sheer density and complexity of these networks is mind-boggling. In old growth forest, with a healthy fungi population, the ground beneath a single footfall may easily contain 5 miles of mycelia if the strands were laid end to end. The complex connectivity of mycelial networks have been compared to the internet, the cosmos and the human brain.
4: Understanding Fungi: Food Sources
Understanding mycelium is the key to understanding fungi – and predicting when and where you might find useful mushrooms. Unlike nearly all plants, fungi cannot manufacture their own food. Webs of mycelium grow outwards from their point of origin, seeking food. Suitable food sources are broken down by enzymes secreted by the hyphal tips, before being absorbed (along with water) through the cell walls. They are then transported throughout the wider mycelium.
Fungal food sources usually come in one of three ways:
– Saprophytic, whereby dead organic matter is broken down. Without saprotrophic fungi breaking down dead vegetative matter (such as lignin, the substance that makes wood such a great building material), forests would be impenetrable masses of fallen trees, branches and leaves. Saprotrophic fungi are crucial in recycling nutrients and building fertile soils. Wood blewits and trooping funnelcaps are examples of good edible saprophytic fungi.
– Parasitic, whereby living organic matter is attacked by mycelia. Parasitism, though often a nuisance to host organisms and humans, should be seen as part of a natural cycle of renewal – older, weaker trees and plants being more susceptible. Many trees live with parasitic fungi for long periods, and in some cases mildly parasitic species can protect the host from more virulent parasites – see, for example, my post on hen of the woods (Grifola frondosa). The most infamous parasitic fungi is honey fungus (Armillaria mellea), which grows aggressively on hard woods and can wipe out large tracts of forest. From a certain perspective, honey fungus can be seen to be harvesting trees: killing them, rotting them down, building soils to allow the next generation of trees to grow, lying dormant until these trees are sufficiently weakened by age, then starting the process again. This technique of tree farming has resulted in the single largest living thing on earth – a single honey fungus mycelia 2.4 miles across with an estimated biomass of 600 tons!
– Mycorhizal. This comes from the Greek words myco (fungi) and rhizo (root), describing a complex, mutualistic relationship between fungal mycelium and the roots of vascular plants. In these relationships traffic through the hyphal tips is two-way, the plants receiving water, phosphorus, nitrogen and zinc in return for energy from photosynthesis in the form of carbon. Carbon sequestration by fungi is crucial in understanding climate science. Many of the finest edible species are mycorhizal. Their complex interdependency with trees explains why they have largely evaded successful widespread cultivation.
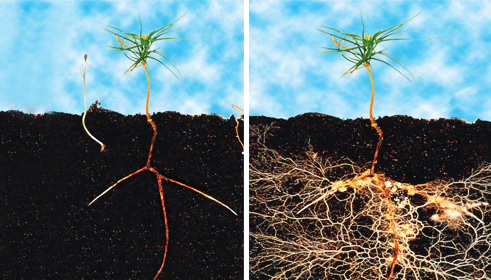
Comparison of fir sapling root (L) with fir sapling with mycelium (R). Uptake of water and trace minerals is hugely increased for the plant. The fungi receives nutrients in return.
Mycorhizal relationships are species-specific: fungi need to be compatible with their partner plants and vice-versa. Some fungi are very faithful, only ever growing with one particular species of tree (eg. the brown birch bolete, Leccinum scabrum with birch), while others are looser in their affiliations (eg. chanterelles, Cantharellus cibarius, which will happily grow with beech, birch, oak, pine and spruce). A nice analogy of this is a trading network consisting of many tribes and cultures. Some fungal tribes speak only one language, so trade with only tribes that speak the same tongue. Other tribes may be bi-, or even multi-lingual, and so trade with many more partners. The most commonly observed mycorrhizal fungi in any given area are likely to be those that trade with the most common tree types in that area – though there are a good deal more variables to add to this equation, some of which I’ve outlined in the graphic below.

Some factors influencing the abundance of fungi. Note that a diverse diet/multiple partner organisms is just one factor. Some mycorrhizal fungi produce a lot of mushrooms after dry summers because the ecosystem services they proved to trees (especially water) has been in high demand, leaving the fungi with abundant resources to invest in reproduction. For a detailed exploration of this subject see my webinar “20 Mushrooms to know before you die”. Graphic ©GallowayWildFoods.com
Some trees, such as the douglas fir, are unlikely to reach full maturity without the correct fungal partners. Nutrient uptake is so accelerated by mycorhizal relationships, that commercial foresters often inoculate saplings with appropriate mycelia. Sycamore and ash trees do not form relationships with any easily observable macro-fungi, so I recommend you focus your mushroom hunting away from them.
The following video gives a great insight into the complexity and importance of mycorrhizal interactions and the Wood Wide Web.
Understanding the sources of food for fungi is a key part of the identification process – in fact before even examining a mushroom, careful note should be taken of the growth medium and which trees are growing nearby.
Mycelium can spread “vegetatively”, permeating suitable substrate with no visible outward signs, provided there is sufficient food – some perfectly healthy mycelia can exist for long periods without producing a reproductive body. A mycologist friend of mine has noted mycelia in the UK that do not produce reproductively viable mushrooms, but who’s DNA matches similar colonies in mainland Europe. He surmises that these fungi have spread entirely through their mycelial growth, and not by spores (see below), and tell of the land bridge and near continuous forest cover that once existed between the UK and continental Europe. While this is an extreme example, many mycologists now view mushroom/spore production in some mycorrhizal species as more of an “insurance policy” than the be-all and end-all of their survival and spread.
Growth, reproduction and survival strategies in the fungal kingdom are just as diverse as those of the plant kingdom, and superficially similar looking forms forms can bely vastly different evolutionary development.
In general, saprobic and parasitic fungi need to grow outwards in search of new food sources and rely heavily on using airborne spore dispersal to reach them, while mycorrhizal fungi can happily exist in one location for many years provided their plant partners remain healthy.

Mushroom rings are the result of the mycelia of saprotrophic fungi moving outward from a point of origin in search of new food sources. This image shows clouded agaric (Clitocybe nebularis).

Mycorrhizal fungi can happily exist in one location for as long as their tree partner is healthy, and may even pre-date its current partner tree, having nurtured its antecedents – fungal networks can be as old as forests. I have been visiting the chanterelle colony in this image for 35 years.
5: Fungi Reproduction: Mushrooms and Spores
When mycelium becomes stressed by environmental factors (eg. a sudden drop in temperature (typically the onset of autumn), meeting an impenetrable barrier (such as the compacted ground of a path), lack of food), and provided it has sufficient water and energy resources at its disposal, hyphae will begin to concentrate and coalesce into hubs, primordia, and ultimately the fruiting bodies we know generally as mushrooms.
When conditions are right, this can happen very quickly, though different species grow at different rates. The cep (Boletus edulis) for example can emerge, grow to a pound or two in weight and start to putrefy within a week. A hedgehog mushroom (Hydnum repandum) on the other hand, may take several weeks to grow to full size, and remain fresh and firm on the forest floor for several more.
Take a deep dive into the science of slower growing mushrooms such as chanterelles and hedgehog fungi here:
The sole purpose of all fungal fruiting bodies is to spread reproductive spores that can carry the fungi to new food sources. Millions of minute spores can be produced by each fruiting body.
From a scientific perspective, the manner in which these spores form and are dispersed is crucial to the general classification of fungi into two key groups (or phyla) – basidiomycota, the spore-droppers, and ascomycota, the spore-shooters. Within the context of this article and for the general field identification of mushrooms, it is not necessary to explore these distinctions beyond the two graphics below, which are discussed at length in my webinar on mushroom identification.
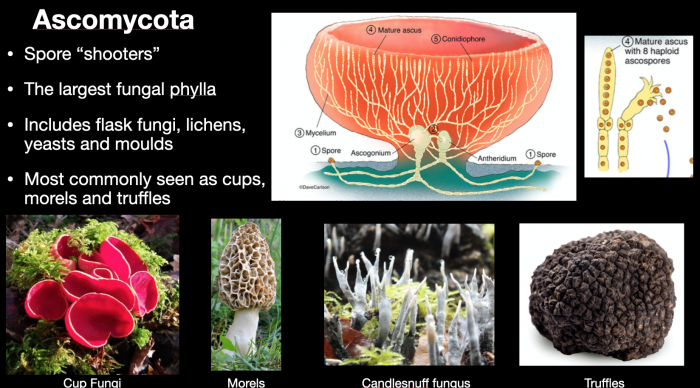
Ascomycota – spore shooters. It is possible to observe large clouds of spores being shot into the air by breathing warm breath onto mature ascomycetes. This cloud is actively ejected as a reaction to changes in humidity, not as a result of you blowing them off. Candlesnuff fungus and cup fungi in particular offer good chances of observing this phenomenon.
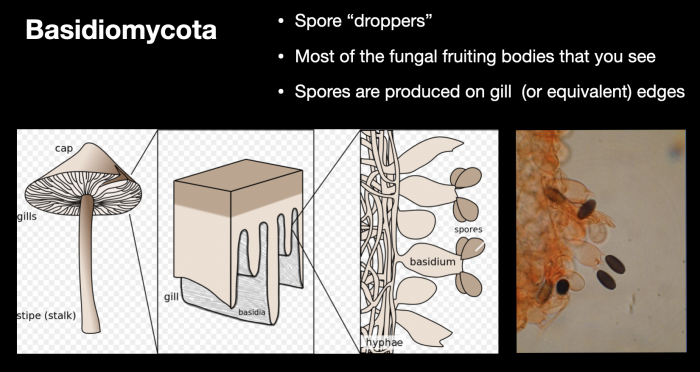
Basidiomycota – spore droppers. These make up the bulk of larger mushroom producing fungi. Spores are usually dropped from basidia (pedestals) on gill, pore or equivalent surfaces, on the underside of elevated caps, allowing them to catch the slightest air current and carry them to new substrates. It has been hypothesised that the variety of tones and colours on the upper side of mushroom caps may play a role in setting up convection currents to carry spores away.

Spore dropping fungi will go to great lengths to keep their spore-bearing surfaces perpendicular to the ground so spores can drop out freely, as demonstrated by this orange birch bolete. This also demonstrates that, despite not requiring light, fungi have in-built “spirit levels”
The astonishing variety of shapes and sizes of fungal reproductive structures reflects the evolution of a vast array of mechanisms for dispersing spores. Classic umbrella-shaped mushrooms generally form then drop their spores through slats (gills) or pores on the underside of the cap. This technique has often resulted from convergent evolution, whereby quite distinct species find similar solutions to shared environmental challenges. This explains why many different species can look remarkably similar to the untrained eye, while having markedly different chemical compositions.

Mushroom gills can range from deep slats, to shallow ridges (as here in this amethyst deceiver), or something more like wrinkles (as with the chanterelles in the background.
A few fungi have developed much more elaborate techniques for spreading their spores, often making them much easier to identify. Stinkhorns produce a foul-smelling coating that attracts flies, which then carry the spores on their feet to other fertile locations. Puffballs fart clouds of spores into the air as their skin is patted by raindrops – giving them their scientific name lycoperdon from the latin lyco – wolf, and perdon – fart. Who says scientists don’t have a sense of humour?

Stump puffball (lycoperdon pyriforme). Puffballs have no gills or pores for spore dispersion. Spores are puffed out like powder from a vent on top when the mushroom is mature.
6: Using Fungi Spores To Aid Identification
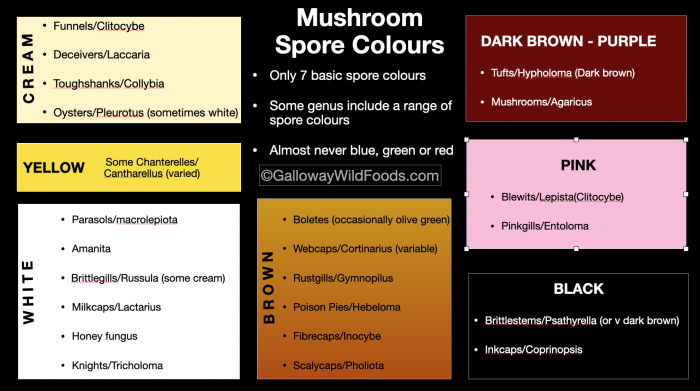
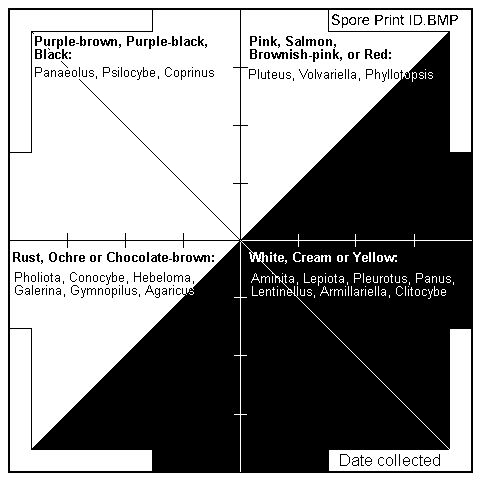
Fungal spores only become visible to the naked eye when they collect in large numbers and usually show up as shades of white, yellow, brown, pink or black. Spore colour is perhaps the single most useful tool you have in moving towards an accurate identification of a mushroom. Though it will almost never tell you exactly what species you are looking at, it will help you to narrow your options down considerably – most good field guides divide mushrooms by spore colour. For example, only about 10% of fungi have pink spores. So instead of randomly leafing through the 300 mushrooms in your field guide, you will be looking at 30. By noting habitat (eg. “Under a beech tree) you may be able eliminate another 15 species before you even start to look at the physical characteristics of the mushroom itself. This process of elimination based on observable characteristics will move you most quickly towards an accurate identification. Randomly looking at photos will drive you mad!
It is sometimes possible to observe spore colour in the field. If mushrooms are growing in tufts, with overlapping caps, spore colour can often be seen on the caps below. Occasionally it can also be seen on grass and other vegetation. Close examination of the gills or pores of a mature specimen may also give an indication of spore colour, but this is not definitive as gills and pores can change colour as the mushroom develops. For example, like cultivated button mushrooms (agaricus bisporus), young field mushrooms (agaricus campestris) have pink gills, which may lead to the conclusion that its spores are pink. However, as the mushroom matures, its chocolate-brown spores start to turn the gills deep brown.
The most accurate way to determine spore colour is to take the mushroom home and make a spore print. To do this, remove the stem and lay the cap gills (or pores) down on a piece of clear glass or paper (preferably half black, half white in case the spores are black or white!). Within a few hours, or better still, overnight, a clearly discernible spore sprint should become apparent. I realise that this process may seem a bit slow and impractical for some, but the investment of this time early will pay dividends in future encounters with the same species – you don’t have to do it every time.
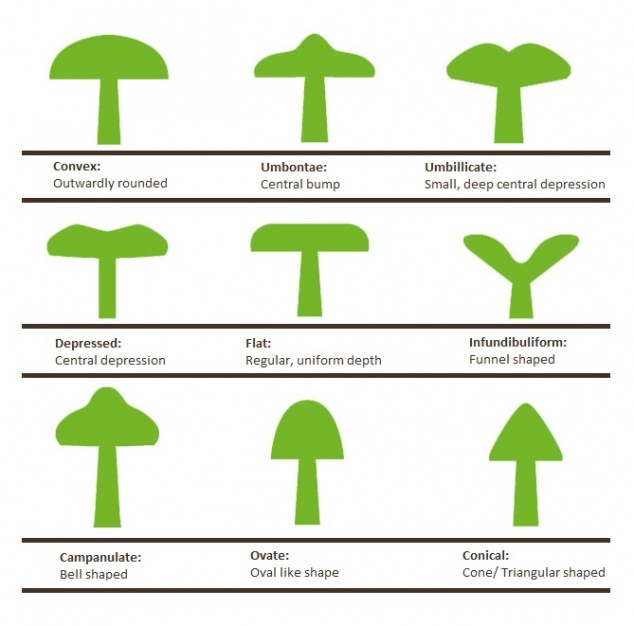
7: The Anatomy of a Mushroom
“The more you see, the more you see there is to see”
To the unpractised eye, many fungi look very much the same. With a little training, a basic understanding of mushroom anatomy, and familiarity with the terminology used in guide books to describe them, once indistinguishable mushrooms can start to look quite different. Think of an alien coming to earth: initially all humans would look very much the same. After spending a little time among us however, it would quickly learn to distinguish our subtly different nose shapes, hair colours, physiques etc to the point where it could tell us apart at a glance. When entering the world of fungi, you are that alien. Spend some time getting to know them!
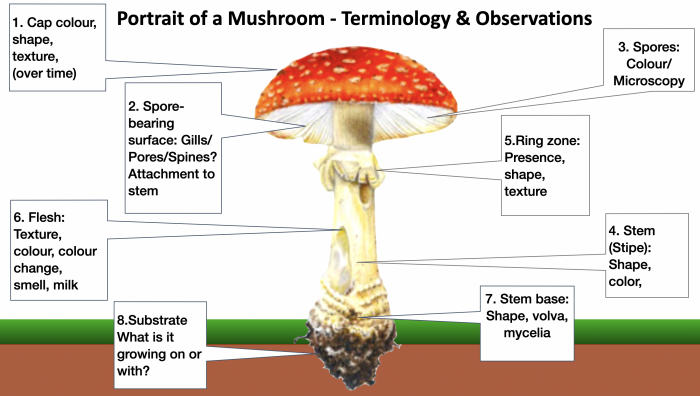
Key mushroom features and observations that will help with identification. This image is from my “Wild Mushroom Identification” Webinar, in which all these and more are discussed at length. I use the fly agaric in this illustration because it exhibits many different features that mushrooms can have. Graphic ©GallowayWildFoods.com
In order to accurately identify mushrooms, close observation is needed, and this almost always requires picking the mushroom. Much debate has gone on down the years in fungi circles as to whether one should pick fungi by cutting the stem, or by pulling and twisting it from the ground. The argument ran, often vehemently, that by “uprooting” the mushroom, the mycelium would be damaged, impairing future growth, in the same way as pulling the root of a plant up may damage its prospects.
The truth is, it depends on the mushroom. Pulling a saprophytic mushroom up may well bring with it a good clump of mycelium, especially if it is feeding on loose material like leaf litter (this observation could be useful later for identification). Mycorhizal mushrooms however, tend to detach quite cleanly from their parent mycelia.
Of course, a novice coming to a mushroom for the first time, won’t know which of these it might be. My advice is to gather as much of the mushroom as possible, including subterranean parts. These can greatly aid in identification, and provided its done sensitively, damage to the mycelium should be minimal. Once familiar with a species, harvesting techniques can be adapted to minimise disruption. Remember too, that even the most toxic mushrooms, unlike some toxic plants, can’t poison you just by handling it for identification purposes. That said, you’ll probably want to wash your hands thoroughly if you discover you’ve been holding a death cap, and mycologists handling them for extended periods of research do wear gloves.
Read my blog: How to Harvest Wild Mushrooms..Cutting v Picking – What’s the difference?
The development of many mushrooms starts with a ‘button’ phase, whereby the immature fruiting body is encased in a universal veil. As the mushroom grows, it bursts out, often (but not always) leaving remnants of that covering attached to the cap and stem base. These can appear as scales, and occasionally a full, bag-like volva remains at the bottom of the stem, often (at least partially) underground. The presence/absence and structure of this can be crucial in identifying members of the amanita family. As this includes dangerously toxic species like the death cap (Amanita phalloides) and destroying angel (Amanita virosa), this is crucial information!
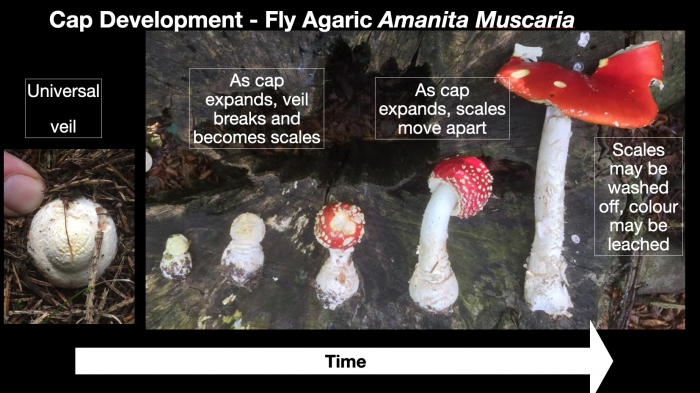
Growth of fly agaric over time, taken from my Webinar “Wild Mushroom Identification”. From button with intact universal veil to fully expanded cap, possibly with scales washed off, can be as little as a few days in optimum growing conditions. This rapid growth and associated change of form is another reason why identifying mushrooms can be quite challenging. Image©GallowayWildFoods.com
Here are some of the key parts of fungal anatomy, with things to look for and some of the terminology used to describe them:
- Cap: look at the size, shape, colour, texture and peelability of the skin. Remember that all these can change quite quickly according to the age, stage of development and environmental factors. For example, colour can be washed out by heavy rain, or bleached out by sun. Young specimens tend to exhibit stronger colours. A good guide book will recognise this development and describe the most likely colour and size parameters.
- Scales on cap: look for flakes and patches of different colour and texture from the cap’s skin. Remember these can be washed off by rain, and become more diffuse as the cap expands. Differentiate between scales and patches where colour has been washed out of the cap by consistent drips from foliage above.
- Stem (often referred to as stipe): Size, colour, shape and texture. Size can be quite variable within species, so often, size in relation to cap can be a more useful observation. Note any colour changes, patterns and textures on the surface, especially scales and net patterns (reticulum). Note whether the stem is swolen at the base (bulbous), tapering or straight, and meticulously check for any sign of a bulb, bag or casement at the base. How fibrous is the base? If it is tough and hard to break, splitting into longitudinal fibres, you may be holding a member of the toughshank (Collybia) family. If its super brittle, this may lead you towards the brittlestem (Psathyrella) grouping.
- Ring: In addition to the universal veil described above, the spore-producing surface under the cap is often protected by a veil in the early stages of growth. As the cap expands, this ruptures and falls away, sometimes (but not always) leaving its remains on the stipe in the form of a ring. Note if a ring is present, and if so, its colour and any markings or striations. Sometimes there may be no ring, but a more or less abrupt colour change in the stem where a ring once joined – this is also useful information. Be very alert that on young caps the veil may fully cover the spore-bearing surface, making gilled mushrooms look like very finely pored mushrooms. This can be a dangerous mix-up and has resulted in some tragic misidentifications. Always give the underside of young caps that appear to have neither gills nor obvious pores a gentle scrape to see what lurks beneath.

Some mushrooms will not have a distinct ring, but colour changes and banding near the top of the stipe can help with ID. These Cortinarius bolaris show the red remains of their web-like veil, placing them in the webcap family
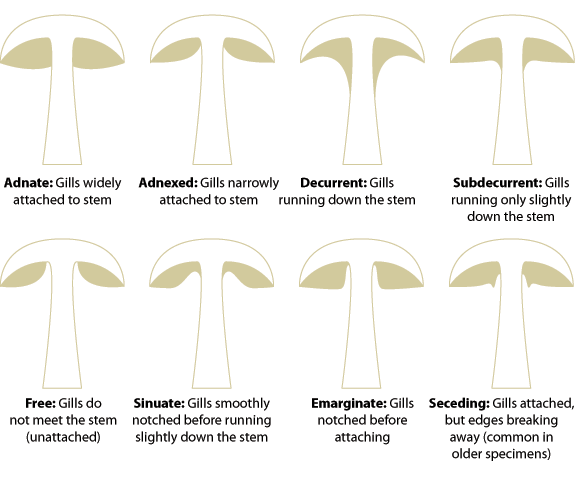
- Under the cap: Gills or Pores? Where gills are present, note their texture (eg. Brittle, waxy, soft), how far apart from one another they are, whether they fork, whether any are partial (ie not running the full radius of the cap), and how they join the stem – do they run down it (decurrent) or hang down free of it – or somewhere between those extremes? Similar observations can be made of pored mushrooms – do the tubes (the ends of which are the pores) grow attached to the stipe, or hang free?
- Flesh: Squeeze, pick at and explore the flesh. Note any textures – spongy, brittle, fibrous etc. Look for any liquids exuding from the broken flesh – a milky (or occasionally more brightly coloured) liquid will help to place it in the milk cap (lactarius) Smell the broken flesh and around the underside of the cap. Many fungi have distinct smells that can be very useful for identification – eg. Curry, iodine, coconut, fish.
- Taste: Perhaps rather shockingly to some people, many guide identification guides contain notes on taste for the purposes of identification rather than gastronomy. This can be very useful, but I don’t recommend it for novices. Tasting should only be used for ID once you have eliminated dangerous families from you enquiries (be absolutely sure you don’t have an Amanita, Fbrecap, Webcap or Gallerina!) Even then, it is important to understand the difference between tasting and eating! Tasting means placing a tiny fragment on the tip of your tongue and waiting for 30 seconds to see if any noticeable flavour develops. If none is noticeable, a gentle chew at the front of the mouth is the next step. The fragment should always be spat out after tasting, and immediately if any hotness, pepperiness or unpleasantness is felt. Be very clear that this is not a test to see if the mushroom tastes “nice” (apparently death caps taste not too unpleasant, though I’m not sure who discovered this!). It is purely a test to find another identifying feature. Note also, that just because an animal or insect appears to have been dining on the mushroom, this does not mean it is safe for human consumption. Unless you have the same digestive system as a slug! Read more on this here.
All these observations together, along with the critical information on habitat and spore colour discussed in detail earlier, should build up an intimate portrait of your fungi, and allow you to make a meaningful identification with a degree of confidence. Bear in mind that the confidence you have in your identification needs to be 100% if you intend to eat the mushroom! Less certain identifications are normal, and a useful part of the learning process. There are very few mushrooms that I bring home for identification that I feel certain of from the first book I look at. Often identification is declared “on the balance of probabilities” – again, not sufficient for eating!
8: Using Fungi Keys
Now you know the features and terms of fungi identification, you need to put it all together…
A key, in this context, refers to a flow chart of questions about the observable features of the mushroom, that eliminate family groups and eventually arrive at an identification – either to genus, or less often to species. They are extremely useful. I confess to being a late-comer to using keys in my exploration of fungi – or rather, to using written keys. In truth, every birder, botanist or mycologist automatically runs through a key in their head on encountering something requiring identification. This can start with something as simple as “Its winter” = “This is unlikely to be an autumn mushroom”. Keying can, and to some extent should, be experience based. But its probably more useful at the start to use a key that an expert has written with someone at your knowledge level in mind as the end user. Most good fungi guides will have a key of some kind. Do try using them, if only to help you compare your find to 30 pictures instead of 300!
See my reviews of fungi guide books, including Peter Nicols’s fungi key.
9. Thoughts on online fungi guides and Apps
There are some excellent websites out there, but there are also some I would not recommend. I suggest you never do all your research based on one website. Certainly don’t rely on a google image search – these are often incorrect. Always check against an authoritative guide book. Never eat a wild mushroom based on the advice of only one website or “expert” on a forum. I get sent dozens of ID requests a week in mushroom season. Where the request is made politely, sensibly, with useful photographs, habitat information, and evidence the asker has invested some of their own time, I (and others like me) offer an opinion on ID where possible. This is not intended, and should never be treated as, a basis for eating a wild mushroom. You have to take full responsibility for that yourself!
Read my in-depth discussion of ID Apps, Online image searches and ID forums here
See my recommended websites and social media for foragers here
Further reading:
- Fungi Webinars
- How To Ask For Help with Identifying Wild Food
- Fungi Identification Apps – Careful Now!
- Recommended Online Foraging Resources
- Review of Fungi Guidebooks
- Edible Wild Fungi Guide
- More Beginners Foraging Guides








10 Comments
This has been really useful and a great read. I have a mixed spruce wood in Herefordshire that I use for my Forest School groups and I have been blown away by the amount of fungi I have seen this Autumn although we dont touch them it’s always interesting to be able to identify specific fungi.
A wonderful article, thanks.
Thank you so much for the clear extensive information in this article. I have learnt a lot reading it and have a broader knowledge as a result. I can add information learn here to my talks on mushrooms in my forest school. Greatly appreciate you sharing this. It’s excellent .
Hello Mark. Great website. Extensive and informative and also entertaining. Just a quick query. I have been reading Peter Wohlleben’s “The Hidden Life of Trees”. In discussing the symbiotic relationship between mycorhizal fungi and their host he states that the mycelium filter out heavy metals from the soil and that these “turn up every fall in the pretty fruiting bodies” Have you any insight into this?
Hi Alex, Yes, it is quite species-specific, but in general fungi are great re-mediators of polluted land due to their absorption of heavy metals, hydrocarbons and even radiation. Obviously this means extra care should be taken when harvesting them for food from on/near polluted sites/busy roads. A key text on the positives of this attribute is Mycelium Running by Paul Stamets.
Thank you Mark for this very informative article. I am a beginner and wi was looking for a good fungi key. I see you mention one here by someone called Peter Nicols. However following the link I can’t find it. Neither can I find it by running a Google search. Could you point me in the right direction? Are they other keys you’d recommend? Thank you
Hi Vincent,
Correction: Paul Nicol, see here:
Cheers,
Mark.
Hi Mark, what a fabulous informative site! My wife and I recently formed an interest in mushroom foraging and on a walk earlier today, found what we believe to be porcini mushrooms.
Slightly nervous about eating them without expert confirmation of what we have here and wonder whether you’re able to confirm from the photos at this dropbox link?
https://www.dropbox.com/sh/6im9bzzpkf6tmsr/AACOKCEpV8uIDNmmUtXstMu1a?dl=0
They were found growing under some pines and appear to have all the appropriate characteristics: dry but slightly tacky caps, firm white flesh, off white pores and vertical markings down the stem with a webbing pattern towards the underside of the caps. I’ve also done a taste test and they have a nice mellow nutty flavour.
The only other issue we have is that there does appear to be some maggot activity, particularly in the caps and some bright yellow discolouration at the base of the stems. Are you able to advise what, if anything we can do about the maggots, what the yellow discolouration is and whether they’re safe to eat?
Many thanks.
Tom
Hi Tom,
Thanks for this. A great example of how to ask for help with ID! I get so many blurry pics with no habitat info that are, frankly, exasperating! This is a breath of fresh air.
These are certainly boletus edulis. Here is my post on them:
Unfortunately fungal gnat larvae are part of the deal with ceps, even the finest young specimens often having some. Its up to you as to how squeamish you feel about it. Entomophagy is the future, and these wee guys have only been eating super-gourmet fungi… The usual way to deal with more than you are comfy with is by slicing and dehydrating.
The yellow discolouration is the early signs of hypomyces – bolete eater fungi – which can render prime ceps piles of mush in under a day. Cut out the corruption. A tiny hint of hypomyces in older specimens actually adds to the flavour of ceps when dried, but mostly you want to avoid it.
People all over the world enjoy these fabulous mushrooms raw and cooked, but the usual rules apply: eat a small amount, well cooked the first time you try, and progress from there. My wife gets a sore tummy from raw cep, and also mostly avoids it cooked. Unlucky her!
Enjoy,
Mark
Hi Mark,
Fascinating reading before tomorrow’s course. Well written for a beginner! Looking forward to the course.